A toolkit for country decision makers, program planners and local implementers
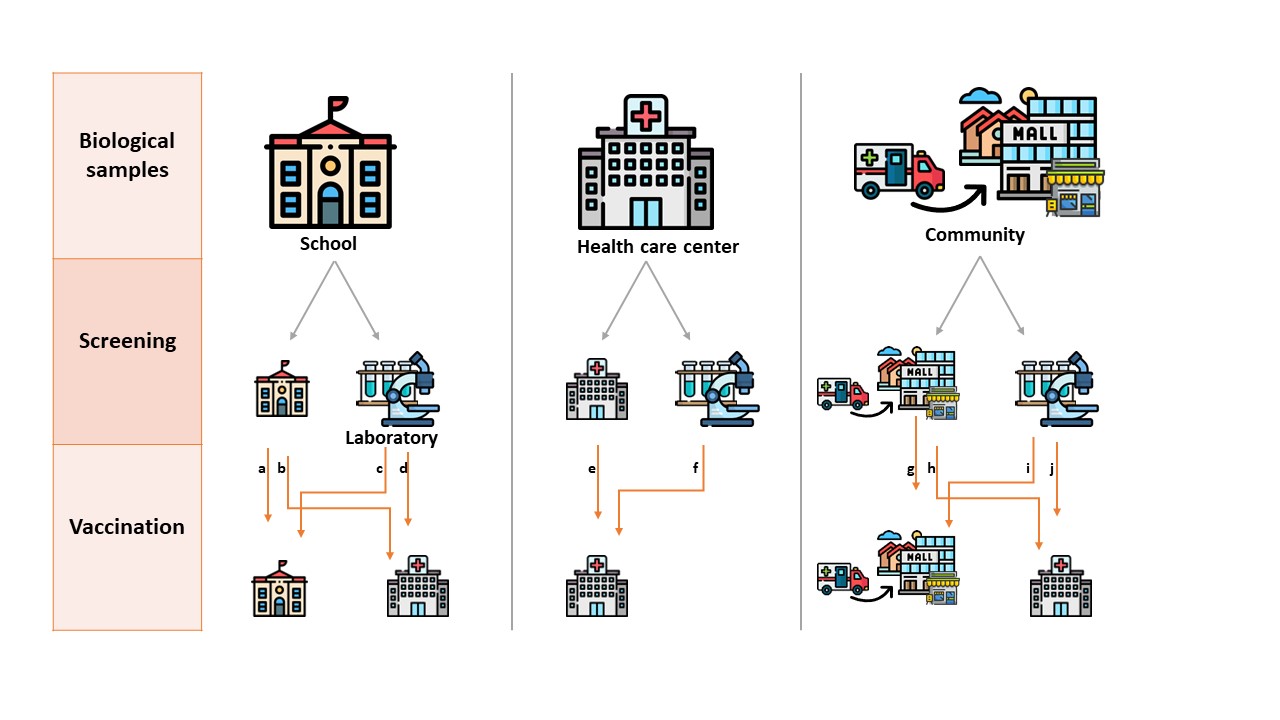
Based on results of long-term clinical trial data confirming differences in dengue vaccine performance based on prior dengue infection, Sanofi Pasteur proposed an update label for Dengvaxia®, the dengue vaccine, and the World Health Organization (WHO) provided revised recommendations1 for its use in endemic countries. The preferred option consider only offering vaccination to those who have documented evidence of a past dengue infection. The resulting Screen and Vaccinate (S&V) strategy considers pre-screening of eligible vaccine recipients using specific rapid diagnostic tests for previous dengue infection.
A range of activities has been developed by EpiLinks to support endemic countries in Latin America and Asia Pacific with this novel strategy for vaccine implementation.
The dengue vaccine implementation toolkit is composed of six modules addressing essentials aspects: General Information; Implementation Strategies; S&V Sessions; Safety; Communication; Logistics.
Each module considers specificities according to possible approaches (Screen and Vaccination in campaigns or routine programs, vaccination of laboratory-confirmed clinical cases), and strategies (different intervention settings, 1-step vs 2-step strategies for Screen and Vaccination).
These modules serve as working tools to deploy preparedness and assessment activities adapted to each local situation. Country workshops and scientific communication activities were carried out, such as a presentation of implementation strategies and challenges at the PDC/GDAC 2020 meeting (Les Pensières Center for Global Health, France). Several scientific articles have been published in Vaccine partly based on these activities2.
1. Revised recommendations
2. Several scientific articles have been published in Vaccine partly based on these activities:
- Implementation strategies for the first licensed dengue vaccine: A meeting report. Fongwen et al., Vaccine, 2021.
- Updated recommendations of the International Dengue Initiative expert group for CYD-TDV vaccine implementation in Latin America. Torres et al. Vaccine, 2019.
- Pre-vaccination screening strategies for the use of the CYD-TDV dengue vaccine: A meeting report. Wilder-Smith et al., Vaccine, 2019.
Ressources (PDF) :
- 1_DENGUE VACCINE TOOLKIT_General Information_November2021 (2)
- 2_DENGUE VACCINE TOOLKIT_Implementation Strategies_July2021(2)
- 3_DENGUE VACCINE TOOLKIT_SandV Sessions_July2021(2)
- 4_DENGUE VACCINE TOOLKIT_Safety_July2021(2)
- 5_DENGUE VACCINE TOOLKIT_Communication_July2021
- 6_DENGUE VACCINE TOOLKIT_Logistics_December2021